Pure Hydration has been working at the leading edge in the development and application of modern technologies for individual water purification (IWP) devices for the last two decades.
In that time we’ve heard and read many claims about the performance of different filtration and purification systems for producing safe drinking water from microbiologically and/or chemically contaminated sources. All too often, these statements hide or simply ignore shortcomings and failures in products, although they are increasingly repeated on other blogs and internet forums with little or no attempt to validate, qualify or even understand the assertions made.
With multiple products employing a variety of techniques in the attempt to provide potable water, it can be extremely difficult to evaluate relative performance by simply attempting to compare the physical characteristics of one equipment type with another that utilises a wholly different technology (or technologies), although that hasn’t stopped people trying to do so.
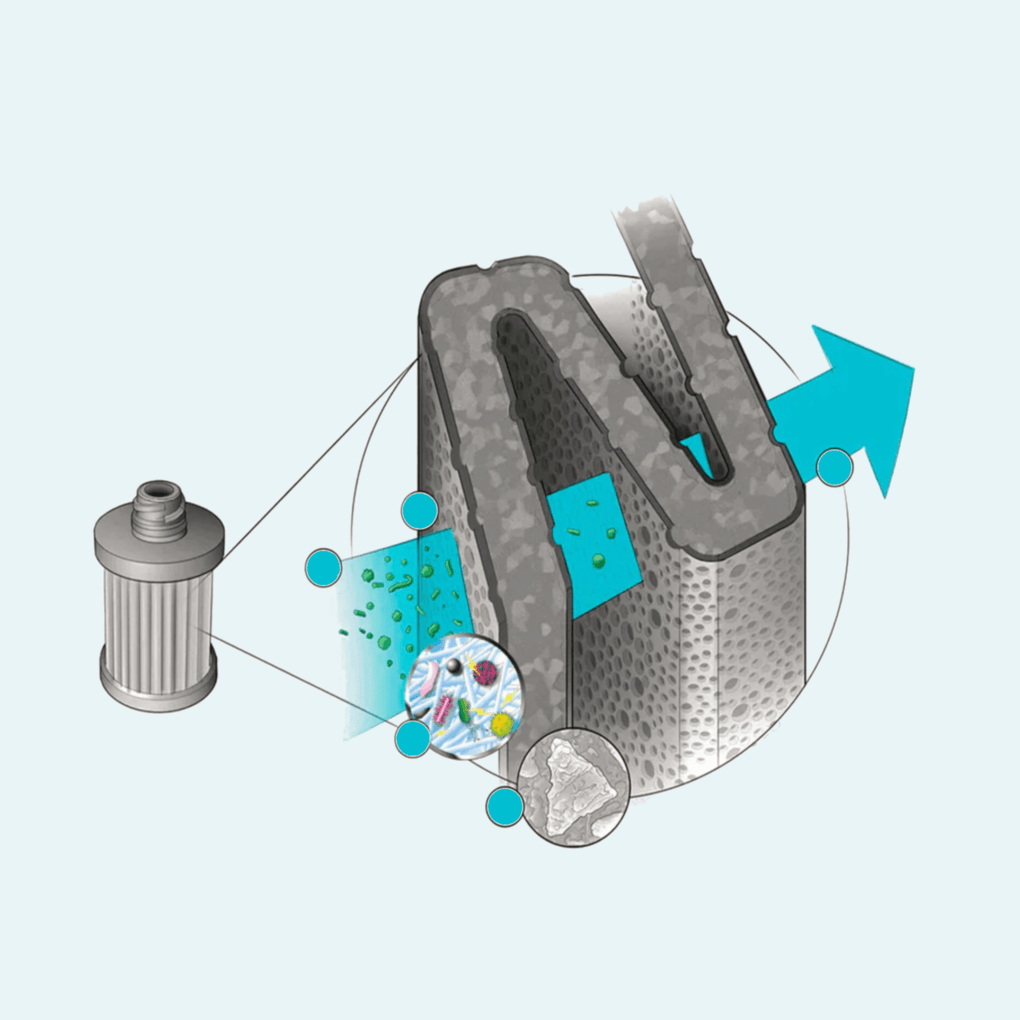
The term ‘pore size’ is frequently invoked in this respect, and is used to characterise a ‘hole’ through which passage by increasingly small particles or organisms is either entirely prevented (‘absolute pore size rating’) or is blocked as a defined percentage of the total contaminant level (‘nominal pore size rating’).This is perfectly acceptable when you are describing a device which uses, for example, hollow fibre membranes as the basis of its filtration capability, and where the ability to block such as harmful E-coli bacteria from passing through a filter is achieved by having an appropriately restrictive pore size, but it is decidedly unhelpful when looking at a technology that deliberately utilizes a larger spacing in its purification matrix to ensure enhanced water flow rates and which removes the offending microbes by other means.
Using the terminology of a single type of old technology (hollow fibre technology was commercially developed in the 1960′s) to define how all new products should be categorized is rather like trying to describe how, for example, a car’s brakes should work without acknowledging the difference between a drum brake and a disc brake, and then disregarding automotive development advances such as ABS.
Furthermore, using only one technology parameter to describe the reduction method for just one category of risk, i.e. the microbiological threat, does not adequately address how performance may be compromised by other physical characteristics of that technology, for example, fouling of the outer surface of hollow fibres, and it does not explain how a product would manage reduction of a chemical hazard in the same water, leaving the purchaser potentially unaware of either the limitations or broader capability in terms of the wider spectrum protection provided by a device.
The M.A.D. ® media is configured in a professionally engineered casing (either in the format of an integrated water bottle cap or as an inline purifier for use with hydration reservoirs or our gravity fed systems) to ensure its integrity is maintained in the toughest of environments.
All Pure Hydration products have been subjected to extensive testing and certification processes.